Bather Loads, Pollution, Turnover...It's All Relative.
August 26, 2016
Relative Pollution
Posted by poolplantcourses.com. Posted In : Pool Water Pollution
Run a Swimming Pool? You Need a PSOP!
August 26, 2016Posted by poolplantcourses.com. Posted In : Health & Safety
Could Your Pool Turn Green, Like The Olympic Pool In Rio?
August 25, 2016
Apparently, an operator added hydrogen peroxide to the Rio Olympic Pool. The rationale behind the decision to add this chemical is not clear. It could have been intentional, as hydrogen peroxide is used in some swimming pools as an oxidiser. The problem is that the chemical is also used as a chlorine neutraliser. So when the hydrogen peroxide was added, it went to work and promptly neutralised all of the chlorine thus allowing organic plant life to thrive, since it now had water, sunlight, nutrition and the absence of a biocide (chlorine).
Could it happen in your pool?
Yes, possibly, but probably not because of the addition of hydrogen peroxide. The pool water is more likely to turn green in outdoor pools because of exposure to sunlight, which contains ultra violet light (UV light neutralises chlorine). Some outdoor pools end up looking more like ponds due to water that is green because of algal growth.
The pool plant operator needs a way of 'stabilising' the chlorine. Cyanuric acid is used for this purpose. Cyanuric acid binds with the hypochlorous acid (the disinfectant element of chlorine) and makes it more resistant to degradation by UV. The drawback is that it also makes the hypochlorous acid less effective as a disinfectant and for that reason, higher free chlorine residuals must be maintained when using cyanuric acid (2.5 - 5.0 mg/l).
Posted by poolplantcourses.com. Posted In : Pool Water Chemistry
Overchlorination - How to deal with it and get the pool open for business again!
July 22, 2016
It may be necessary to decrease the levels of chlorine on
occasion and certainly following superchlorination. If you are going to be
dumping a significant quality of swimming pool water for any reason, there
would usually be a requirement to let the local water authority know and they
would almost certainly require you to eliminate all traces of chlorine from the
water before they granted permission to discharge (chlorine is harmful to
aquatic organisms).
In normal operations, it would usually be better to bring the chlorine levels down by simply diluting the swimming pool with fresh water. This is safer and would contribute to less chemical pollution as well.
If you do need to decrease the chlorine quickly though, the chemical to use is sodium thiosulphate. The principle to bear in mind is that it takes 5g of sodium thiosulphate to neutralise 1g of chlorine. So if, for example, you had 10.00mg/l of chlorine in a 300m3 pool, that equates to 3000g of chlorine in the pool, since each m3 would have 10g of chlorine in it, and 300m3 X 10g = 3000g. The simplest thing to do would be to calculate how much sodium thiosulphate you would need in order to decrease the free chlorine level by 1.00mg/l. See he worked example below:
300g chlorine X 5g sodium thiosulphate = 1500g
So, in this particular example of a 300m3 pool, it would take 1500g of sodium thiosulphate to reduce the free chlorine level by 1.00mg/l/
From here, the same steps can be taken as given above in order to create a jug for the purposes of hand-dosing sodium thiosulphate (different jug – NEVER mix chemicals). Then, just add the required number of jugs in the same way as for adding calcium hypochlorite. So, in the example given, we would be adding 8 jugs of sodium thiosulphate in order to get the free chlorine down from 10.00mg/l to 2.00mg/l.
Posted by poolplantcourses.com. Posted In : Pool Water Chemistry
Superchlorination - The Correct Way
July 21, 2016
Following on from our previous post regarding hand-dosing of chlorine, here is some guidance on superchlorination.
Superchlorination is not recommended as a routine or even
occasional method of shock dosing to compensate for inadequacies in pool
treatment. It is generally bad practice, and can generate unwelcome by-products.
But if something has gone wrong – poor results from microbiological testing
perhaps, or a catastrophic breakdown in treatment – it may be necessary to
superchlorinate. It can also be a way to deal with contamination by diarrhoea,
as some intestinal pathogens (eg Cryptosporidium) are resistant to normal
levels of chlorine residual. In this case it may be needed where filtration is
inadequate (high-rate for example, or regular coagulation not practised).
Superchlorination can also deal with other organisms should the need arise.
Two pool parameters are needed as a starting point:
· the capacity of the pool in litres. Note: 1m3 = 1,000 litres, 1 gallon = 4.54 litres
· the pool turnover period (the number of hours for a volume of water equivalent to the entire water volume of the pool to pass once through the water treatment plant).
The following chemicals and equipment will be required to undertake the procedure (which of course must include subsequent dechlorination):
· a suitable chlorine donor – sodium or calcium hypochlorite (not any cyanurate-based disinfectants as they are not effective enough)
· a dechlorinator – normally sodium thiosulphate pentahydrate
· a pool water test kit together with a dilution pot
· one or more clean 10-litre plastic buckets
· a cold water supply.
Operators must be confident that the pool plant (valves, seals etc) will withstand superchlorination.
Step 1. Close the pool to swimmers. If more than one pool uses the same filtration system, all pools will have to be closed to swimmers and superchlorinated. Do not allow anyone to enter the pool(s) until superchlorination and subsequent dechlorination is completed. Isolate automatic dosing controllers to avoid damage to the sensors.
Step 2. Raise the free chlorine concentration as required to deal with the problem, based on the chart below.
|
Reason for superchlorination |
Concentration required mg/l |
Contact time |
|
Diarrhoea (possible contamination with Cryptosporidium |
20 |
13 h |
|
Algal growth |
10 |
24 h |
|
Legionella (spas) |
50 |
16 h |
|
Raised colony counts, coliforms, E. coli |
5 |
1 h |
|
Raised P aeruginosa |
5 |
12 h |
Step 3. Add the total amount of calcium hypochlorite to tap water in the bucket(s) until fully dissolved/mixed. Then spread evenly around the pool surrounds and mix well by agitation. Failure to dilute and spread evenly can result in the precipitation of hardness scale. Superchlorination will raise pH, so acid will be needed to reduce the pH value to 7.5 or less. In the case of contamination by diarrhoea ensure the water temperature is 25°C or higher.
Step 4. Ensure that the filtration system is operating while the water reaches and is maintained at the chlorine level required for superchlorination (see Table). With spas, all aerators, sprays etc. should be operating throughout.
Step 5. Test the free chlorine concentration 15 minutes after the initial addition to ensure that the correct concentration has been achieved (see Table). This may necessitate dilution of the sample with chlorine-free water to give an accurate measurement.
Step 6. Leave for the desired contact time (see Table). Check every two hours to ensure the concentration is being maintained. If necessary re-dose to reinstate the required free chlorine residual, again checking pH.
Step 7. Backwash the filter thoroughly after the given contact time and top up pool to the required water level. Be sure the backwash effluent is discharged directly to waste (and not to a septic tank or water course). As usual, rinse the filter before resuming filtration.
Posted by poolplantcourses.com. Posted In : Disinfection
How to Hand-Dose Chlorine...The Right Way!
July 14, 2016
Sometimes there will arise a need to introduce chemicals into the pool manually (hand-dosing). This is a potential hazardous activity and should not be performed by people who have not received the appropriate level of training.
General Procedures
• Always wear the appropriate PPE.
• Always add the chemical to the water, NEVER add water to the chemical.
• NEVER mix a chemical with another chemical. Only ever mix with water.
• Never hand-dose chemicals into the swimming pool when occupied.
• Always allow time for thorough mixing and distribution of the chemical into all areas of the swimming pool water.
Increasing Chlorine
The following method will outline how to add a hypochlorite disinfectant to the swimming pool. If you’re using a chlorinated isocyanurate disinfectant, follow the manufacturers’ instructions as the method will be different.
We recommend using calcium hypochlorite granules for the purpose of hand-dosing. It’s safer to store and handle than sodium hypochlorite.
Step 1. The first thing you need to do is calculate how many cubic metres of water you have in your swimming pool. Do this by multiplying the length by the width by the average depth. See the worked example below:
Length (20m) X Width (10m) X Average Depth (1.5m) = 300m3
Step 2. The next thing to do is calculate how much calcium hypochlorite granules you need to add in order to increase the free chlorine reading by 1mg/l. Do this by dividing the pool volume figure (from step 1.) by 0.65. The reason you need to divide by 0.65 is because calcium hypochlorite is typically only 65% chlorine. Some products are 70% chlorine, in which case you would divide by 0.70. See the worked example below:
300m3 / 0.65 = 462
The figure obtained provides you with the amount of grams of calcium hypochlorite granules you need to add to the swimming pool in order to increase the free chlorine reading by 1.00mg/l.
Step 3. Use a set of kitchen scales to measure out 462g of calcium hypochlorite granules into a clear plastic jug.
Mark a clear line on the jug to indicate the level of calcium hypochlorite granules at 462g.
Step 4. Decide how much you need to increase the free chlorine reading by. For example, if you have zero free chlorine in the pool and you would normally operate at 2.00mg/l, then you need to increase by 2.00mg/l. This equates to the number of jugs of calcium hypochlorite granules you need to add to the swimming pool, i.e. 2 jugs.
Step 5. Now you need to add the granules to the swimming pool water. This can be done by carefully depositing the granules into either the overflow channel (in a deck-level pool) or the skimmer baskets (in a skimmer-basket pool). From here, the granules will be drawn into the balance tank (if there is one), or directly into the suction-side pipework of the circulation system.
Step 6. Allow some time for the granules to dissolve and make their way around the system and into all areas of the swimming pool. How long this will take will be dependent on a number of factors, such as the efficiency of the system hydraulics.
Step 7. Carry out a set of pool tests, taking the sample from a point in the swimming pool as far as possible from the inlets. This is to help you determine whether the chlorine you have introduced has been distributed to all areas of the swimming pool. If necessary, carry out further tests in order to be sure that all areas of the swimming pool have a sufficient level of disinfectant. Once you are satisfied of this, you can open the pool again to bathers.
Posted by poolplantcourses.com. Posted In : Disinfection
Pool Managers - Do You Know How Your Filters Actually Work?
June 23, 2016
Filtration is an important element of effective pool water treatment. The basic principle is that the untreated water is passed through a filtering medium (such as a bed of sand). The water is able to pass through the gaps between the grains of sand (called ‘pores’), but anything larger than the pore size is trapped within the filtering medium.
- Mild steel
- Stainless steel
- Plastic
- Concrete
The basic sand filtration process work as follows:
Filtration Rates
Posted by poolplantcourses.com. Posted In : Coagulation & Filtration
Swimming Pool Outlets Can Kill!
June 17, 2016
This week, the Royal Life Saving Society published a news article concerning the risk of drowning via suction entrapment in hot tubs.
If the blockage is a person, then tragic consequences can occur, including drowning, disembowelment and transanal evisceration, which is where internal organs are forcefully drawn out through the anus.
- Emergency cut-off devices that automatically turn off the suction pumps when an increase in suction force is detected
- Multiple outlets being fitted so that even if one of the outlets gets covered, the remaining outlets take the increased water flow and prevent a vacuum being created at the blocked outlet. The distance between outlets should be a minimum of 2m.
- Outlets being designed so that it is impossible to cover them and form a seal. This can be achieved via having the grill surface area of sufficient size (the outlet should have a surface area greater than 1m2).
- It can also be achieved by the use of outlets that are designed to prevent a seal being formed around them when they are covered. These are called anti-vortex drain covers. Some examples below.
- Installing a break tank between the pool tank and the circulation system. The break tank is gravity fed, so there is no risk of being exposed to the suction of the circulation pump(s).
- Ensuring that the water velocity through each outlet is 0.5m/s or less.
- All outlets should be fitted to a sump where the outlet pipe is located a distance 1.5 x the pipe diameter from the grid.
- To prevent finger and toe entrapment the gap in the grille covering the outlet shall be a maximum of 8mm.
- Ensuring that all outlet fittings and fixtures comply with BS EN 13451–1 and 3.
Posted by poolplantcourses.com. Posted In : Health & Safety
Is the commercial swimming pool sector regulated? YES (but not very well)!
May 25, 2016
Whilst there is no specific legislation covering the management of commercial swimming pool water, there is plenty of general legislation that is applicable. Read on for a quick primer.
Management Responsibility
It shall be the duty of every employer to conduct his undertaking in such a way as to ensure, so far as is reasonably practicable, that persons not in his employment who may be affected thereby are not thereby exposed to risks to their health or safety.
It shall be the duty of each person who has, to any extent, control of premises or of the means of access or egress or of any plant or substance in such premises to take such measures as it is reasonable for a person in his position to take to ensure, so far as is reasonably practicable, that the premises, all means of access or egress available for use by persons using the premises, and any plant or substance in the premises or, provided for use there, is safe and without risks to health.
If people working under the control and direction of others are treated as self-employed for tax and national insurance purposes, they are nevertheless treated as employees for health and safety purposes. It may, therefore, be necessary to take appropriate action to protect them. If any doubt exists about who is responsible for the health and safety of a worker, this could be clarified and included in the terms of a contract. However, a legal duty under Section 3 of HSWA cannot be passed on by means of a contract and there will still be duties towards others under Section 3 of HSWA. If such workers are employed on the basis that they are responsible for their own health and safety, legal advice should be sought before doing so.
- assess the risks in their workplace
- use competent help to apply health and safety legislation
- establish procedures to use if an employee is presented with serious and imminent danger
- co-operate and co-ordinate health and safety if there is more than one employer in a workplace
- help people to understand what the law says including, for example, how requirements based on EC Directives fit with those under the Health and Safety at Work Act
- help people comply with the law
- give technical advice
The HSE may issue a guidance note together with an Approved Code of Practice (ACoP), or independently. Guidance notes contain practical advice and sound suggestions, and are frequently more informative than the related ACoP. The HSE aims to keep guidance up to date, because as technologies advance, workplace risks and appropriate control measures change too.
Posted by poolplantcourses.com. Posted In : Health & Safety
Can you smell chlorine? Then it's probably a badly managed swimming pool.
May 23, 2016
When the disinfectant gets into the pool water, the free chlorine contained within in immediately gets to work and starts combining with pollution. Once chlorine combines it hangs around in the pool water and is no longer effective as a disinfectant and is now actually more of a pollutant itself. It needs to be removed from the pool by a combination of dilution and filtration.
- Get people into the habit of taking a shower before swimming so that there is less pollution available for the chlorine to combine with;
- Dilute the swimming pool water with enough fresh water (30 litres per bather, per day);
- Ensure enough chlorine is being dosed into the swimming pool (an automated system is always recommended for any type of commercial facility);
- Make sure that the pool turnover time is fast enough for your type of pool;
- Make sure you’re not overloading your pool.
- Keep the pH within the recommended parameters.
Posted by poolplantcourses.com. Posted In : Disinfection
Not bothering with water balance tests? You're asking for trouble!
May 19, 2016
Balanced water testing is something that swimming pools should be doing weekly in order to determine whether pool water is 'balanced'. This refers to whether the water is corrosive or scale-forming, or neither (balanced).
- Calcium hardness
- Total alkalinity
- Pool water temperature
- Total dissolved solids

Increase the levels of total alkalinity, by adding sodium bicarbonate
Reduce the TDS levels (if they are particularly high) by diluting with fresh water.
Posted by poolplantcourses.com. Posted In : Pool Water Chemistry
Got a Spa, Hot Tub etc.? You need to know about legionella.
May 13, 2016
Legionella is a type of bacteria that is of particular concern to the pool operator, or indeed, any operator of a facility that has a hot and cold water system. The legionella bacteria causes legionairres disease, which is an infection of the lungs. The mortality rate is currently 12%, which means that if 100 people were to contract the disease during an outbreak, approximately 12 of them would die.
Recent Outbreak
The report found that had not been filtered or cleaned for weeks, causing the water to stagnate and leading to the formation of bacteria and build up of Legionnaires' disease droplets. When the hot tub was then turned on it is believed the particles became airborne and spread around the garden centre.
Posted by poolplantcourses.com. Posted In : Health & Safety
Chlorine Gas Leaks - Pool Operators Beware
April 26, 2016
Swimming pool operators ought to be aware that it is possible to create highly toxic gaseous chemical substances if something goes wrong in the plant room. For example: mixing calcium hypochlorite (an alkaline substance that contains chlorine) with an acidic substance (like sodium bisulphate - which is commonly referred to as dry 'dry acid') will result in a reaction that will produce chlorine gas.
The above scenario may happen because an operator inadvertently introduces a chemical into the incorrect day tank. This has actually happened in numerous swimming pools in the UK. Procedures need to be in place to prevent this type of scenario. Staff training, selection, use and storage of chemicals, layout of the plant room and labelling of tanks and chemical feed lines are some examples of areas to scrutinise.
Another, perhaps less obvious thing to consider is the circulation system. Specifically - the hazards and risks that can be introduced when the circulation system stops (whether this be on purpose, or because of a system failure). When the circulation of the pool water stops, what should also happen is that the automatic dosing of all chemicals into the circulation pipework should also stop. This would usually be achieved by a electrical switch that is interlocked with the water flow sensor. If its working properly, this safety feature should work when need to stop the dosing of chemicals as soon as the main pool water circulation stops.
If the chemical doing does not stop, and chemicals continue to be injected into the circulation pipework while the water in that pipework is stagnant, there is the possibility that hazardous gases can be created as a product of chemical reactions. These gases could contain high levels of aggressive chloramines (chlorine + ammonia), or even worse, chlorine gas (as a result of the chlorine disinfectant coming into contact with the acidic pH correctant).
In order to minimise these risks, careful consideration should be given to the appropriate placement of chemical injection points (the further apart incompatible chemicals are injected, the better). There also needs to be a procedure in place to cover restarting the circulation system back up again after a stoppage. Pools should be clear of bathers (including the pool hall and changing rooms) when the circulation system is restarted. This is because if there were chemicals dosed into the system while circulation was halted (auto-shut-down systems can never be regarded as 100% reliable), and hazardous gases have been created in the pipework - the gasses will be introduced into the pool area when the circulation is started back up, potentially gassing anyone exposed.
Pool operators are urged to ensure that this issue is given due consideration by way of a suitable and sufficient risk assessment.
Posted by poolplantcourses.com. Posted In : Health & Safety
What is pH and Why is it so Important?
April 25, 2016
pH stands for the 'power of hydrogen' and is a critical factor in the treatment of pool water. The recommended range for the pH level to be maintained at is 7.2 - 7.4. The reason that the pH level needs to be kept between these values is that the disinfection efficiency of the chlorine falls off significantly at higher pH levels and the coagulant will also not be as effective. At lower pH values, the pool water will be too corrosive.
The effect of the pH level on the disinfection process is an area that many pool plant operators fail to fully understand. Therefore, they don't take the correct actions and end up with low quality swimming pool water and an excessive yearly spend on chlorine. Let's take a look at what's going on with pH and chlorine:
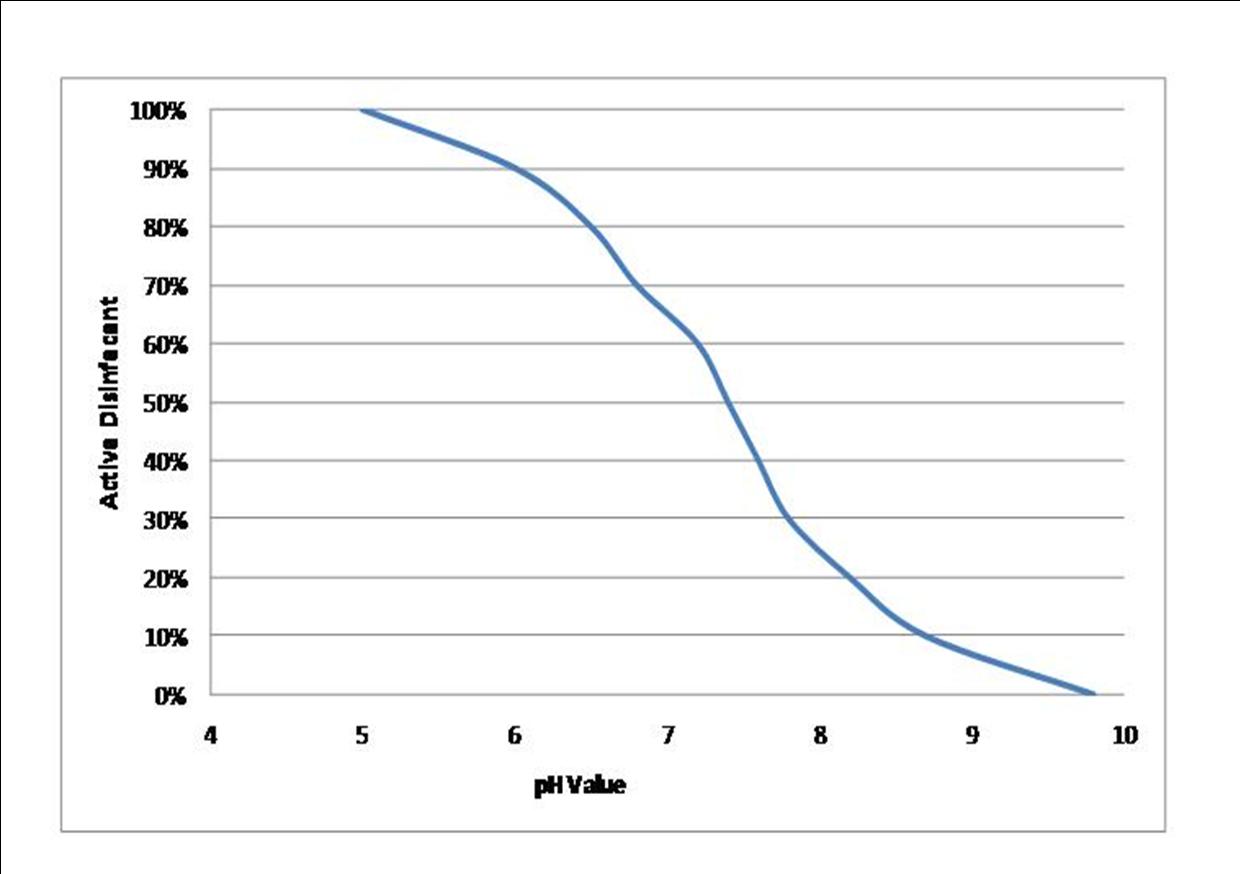
The blue line is the percentage of active disinfectant in the chlorine and as you can see, the percentage of active disinfectant is dependant on the pH level. When you add chlorine to the swimming pool water, chemical reactions start to occur. The chlorine reacts with the water and ends up producing hypochlorous acid and hypochlorite. The key disinfectant in chlorine is hypochlorous acid, which is about x100 stronger than the hypochlorite, so that's what we want more of. The higher the pH level, the higher the proportion of hypochlorite, the lower the pH level, the higher the proportion of hypochlorous acid. At a pH level of 7.4, you've got about 60% of the chlorine as hypochlorous acid, so if your free chlorine reading was 1.0 mg/l when tested, in real terms the amount of active disinfectant would only be around 0.6 mg/l. If the pH level was allowed to get to 7.8, then only 30% of the chlorine would be hypochlorous acid, so if the test reading came out at 1.0 mg/l again, the actual amount of active disinfectant would only be 0.3 mg/l, which would be too low for adequate disinfection.
You need to bear in mind that the free chlorine reading includes both the hypochlorous acid and the hypochlorite, but it does not tell you the proportion of each. This is why it's so important for pool plant operators to understand how and why the pH levels have such a dramatic effect on the disinfection process.
Posted by poolplantcourses.com. Posted In : Pool Water Chemistry
Emptying Swimming Pools
April 25, 2016
The first thing to consider before going ahead with this task is whether it is really necessary to empty the pool at all. Many repairs to the pool lining and/or tiles etc. can be carried out by trained divers, without the need to empty the swimming pool at all. However, there are occasions where the pool water will need to be emptied. An example would be if any broken glass somehow found its way into the pool water. Because glass is completely invisible when submerged in water, the entire pool contents would need to be emptied and a thorough clean-up operation carried out to ensure that all traces of glass have been removed.
Posted by poolplantcourses.com. Posted In : Construction
Gross Microbiological Contamination
April 25, 2016
All commercial swimming pools should be getting the pool water tested at a UKAS-accredited laboratory for microbiological contamination. In most pools this should be done on a monthly frequency, but certain pools, such as hydrotherapy pools, should be done on a weekly basis. The four standard tests and the acceptable levels for each are:
- Aerobic Colony Count > 10cfu/ml
- Total Coliforms >10cfu/100ml
- E. Coli >1cfu/100ml
- Pseudomonas Aeruginosa >50cfu/100ml
- Greater than 10 E.coli per 100ml in combination with an unsatisfactory aerobic colony count (>10 per 100ml) and/or an unsatisfactory P.aeruginosa count (>10 per 100ml)
- greater than 50 P.aeruginosa per 100ml in combination with a high aerobic colony count (>100 per ml)

Posted by poolplantcourses.com. Posted In : Pool Water Pollution
"Do I Need To Send My Staff On A Pool Plant Course?"
December 28, 2015Posted by poolplantcourses.com. Posted In : Frequently Asked Questions
Use a bucket to discover if your pool has a leak
January 24, 2013
If you suspect your pool has a leak, but need to know for sure, you can find out very easily by using the 'bucket-test'. Put some pool water in a bucket and then put the bucket in the pool on the top step. Get it so the level of the pool water is exactly level with the water in the bucket. Leave it for a couple of days and then compare the water levels. If there is a leek, the level of the pool water will be lower than the water level in the bucket.
Posted by poolplantcourses.org. Posted In : Useful Tips
Cryptosporidia
June 12, 2012
Cryptosporidia is a parasite that is of particular concern for pool plant operators because it is not killed by chlorine. The parasites live inside a protective shell called an oocyst which protect them from the chlorine in the swimming pool or spa water. If these oocysts are ingested by swallowing contaminated water, the cryptosporidia with hatch out of the shells and reproduce, causing a gastro-intestinal illness. When the newly-created oocysts are expelled from the body via the faeces, the whole cycle starts again.
As chlorine is an ineffective defence, the pool plant operator must use other methods. The key operational defence is keeping cryptosporidium out of the swimming pool in the first place. Anyone who has been ill with diarrhoea must not go swimming until they are symptom-free for at least 14 days. We recommend putting up some signage about this at reception as well as in the changing rooms etc. You also need to ensure the effective coagulation and filtration of the oocysts. The thing to bear in mind is the fact that without the addition of a coagulant (such as poly-aluminium chloride) to the circulation system at the correct dosing rate, the oocysts will pass straight through the sand in a commercial swimming pool filter. This is because the oocysts are about 5 microns in diameter, whereas the gaps between the sand grains are about 10 microns in width in a ripened sand filter. The addition of a coagulant will cause the minute particles of pollution (including the cryptosporidium oocysts) to clump together to form what are know as 'flocs'. These flocs are large enough so as not to pass through the sand filter and end up in the swimming pool.
Ultra violet radiation and ozone disinfection have been found to eliminate cryptosporidia, but even when using these types of disinfection processes, the use of a coagulant is still recommended.
It is vital that pool plant operators keep their sand filters clean and well-maintained. This means that for swimming pools, the sand filters should be backwashed at least weekly, or according to the filter manufacturers instructions. Spa pool filters should be backwashed every day.
If you end up with loose, runny stool in the swimming pool, you will need to assume that cryptosporidia is present and clear the pool and keep it closed for 6 turnover cycles. While your closed down, backwash the filters, get the chlorine up at the high end of the acceptable range and get the pH at the low end of the acceptable range. Also, scrub, sweep, brush, squeegy, net, hoover the whole area before re-opening.
Posted by poolplantcourses.org. Posted In : Pool Water Pollution
...you can make chlorine from salt?
February 27, 2012
The benefits of this type of system are that there is no direct handling of hypochlorite chemicals and there will be reduced amounts of chloramines (combined chlorine). Some disadvantage of the system are that it produces hydrogen gas, which is explosive and the equipment required can be expensive.
Tweet
Posted by www.poolplant courses.org. Posted In : Did You Know...
The Most Effective Pool Water Treatment Method Ever!
February 22, 2012All sorts of things can go wrong with swimming pool water for any number of different reasons. Things can get out of hand very quickly if you don't know what you're doing. However, there is one key variable that can have a dramatic influence on the quality of your pool water. Get this issue right, and you will experience far fewer problems with regard to pool water quality, you'll spend far less money on expensive chemicals and you and your duty managers will spend far less time trying to resolve issues. What's more, to implement and control this issue is very easy and simple to do and will hardly cost you anything in time, money or effort. So, what is this issue that can have such a dramatic effect? Pre-swim showering!
I know...you're probably feeling a little bit disappointed because after the build-up, you were expecting this article to discuss some fantastic product, new on the market, that would be the answer to your pool water treatment problems. Sorry about that. But really, you should look at your pre-swim showering policy and consider the following facts:
#1. the pollution on bather bodies is what makes up most of the pollution into the pool
#2. the remainder of the pollution only exists in the first place because of the chemical reactions resulting from the chemicals your are having to add to deal with #1.
#3. pre-swim showering removes most of the pollution on bathers
#4. less bather pollution = less chemicals = less chemical by-products = better pool water = happy bathers
How difficult would it be to ensure that every bather, without fail, showers before entering your pool? It is, after all, your pool and your responsibility to keep it clean and safe.
Posted by poolplantcourses.org. Posted In : Useful Tips
Relative Pollution
February 21, 2012Contrast this situation with a spa pool. A spa will only hold about 3 - 10 cubic metres of water, depending on the type. Let's say we have a spa pool that holds 5 cubic metres and has 10 people in it. Each person now has only half of one cubic metre of water each.
Even though there are more people in the swimming pool (and therefore more total pollution), the relative pollution is higher in the spa due to the fact that as a percentage of volume, the pollution levels are higher. The spa is said to have higher relative pollution levels. Spa pools are not the only types of pool that suffer from high relative pollution. Any pool, that has an unfavourable pollution to water ratio will also have high relative pollution. Examples are:
- paddling pools
- splash zones
- teaching pools
- hydrotherapy pools
Posted by poolplantcourses.org. Posted In : Pool Water Pollution
Breakpoint Chlorination
February 13, 2012Imagine a swimming pool that has high levels of pollution. If you were to introduce some much-needed chlorine into the pool, it would quickly end up as combined chlorine as it literally combines with bacteria etc. and becomes useless as a disinfectant as soon as it does so. In fact, combined chlorine as now classed as pollution and it's combined chlorine that makes peoples eyes sting. So, in a nutshell; combined chlorine is something we want as little of as possible, as close to zero as we can possibly get it and certainly no more than 1mg/l.
As all the chlorine we have introduced has become combined chlorine, we need to add some more. We always need to have some chlorine available in order to quickly neutralize contamination. This is referred to as 'free chlorine'. This is the good stuff and we want it in our swimming pool. To be precise, we need the free chlorine levels to be at least double the combined chlorine levels. Free Chlorine is measured with the DPD 1 test, Total Chlorine is measured with the DPD 3 test, and Combined Chlorine is the difference between the two.
But, if the chlorine we introduce turns into combined chlorine, how are we, as pool plant operators, supposed to maintain twice the amount of free chlorine than combined chlorine? Well, that's simple:
- Get people into the habit of taking a shower before swimming so that there is less bacteria available for the chlorine to combine with;
- Dilute the swimming pool water with enough fresh water (30 litres per bather, per day);
- Ensure enough chlorine is being dosed into the swimming pool (an automated system is always recommended for any type of commercial facility);
- Make sure that the pool turnover time is fast enough for your type of pool;
- Male sure your not overloading your pool.
Posted by poolplantcourses.org. Posted In : Disinfection
Latent Heat Transfer
January 23, 2012Both of these forms of heat are used to achieve energy efficiencies in some air handling systems for swimming pool halls. In order to provide a healthy and comfortable environment for bathers, spectators and staff, it is necessary to introduce fresh air into the building. Continually recirculating the air would lead to poor environmental conditions. In order to make way for the incoming fresh air, some of the air already in the pool hall will need to be expelled. The fresh air will need heating and it is possible to obtain some of the energy necessary for this from the outgoing air, which is hot and contains much more moisture than the incoming air (at least in the UK anyway).
What you may find surprising is that both the sensible heat (the difference in temperature) AND the latent heat (the difference in state, ie, liquid to water vapour) can be transferred. The video below provides a good explanation and demonstration as to how this happens. It demonstrates how energy is released when water turns to ice, but the priciples are the same when water vapour (in the outgoing air) is turned into water (as it condenses onto the condensing coil in the air handling system).
Posted by www.poolplantcourses.org. Posted In : Videos
Personal Protective Equipment (PPE) Assessments
January 23, 2012- Download (free) HSG53 - Respiratory Protective Equipment at Work.
- Get the MSDS sheet for the substance in question (go here for calcium hypochlorite MSDS).
- Work through the steps. There are several worked examples in the guidance, but for a worked example for the task described above go here.
Can the risk be completely eliminated, if not;
Can the risk be reduced (using a less hazardous substance);
Can the person be isolated from the task (mechanising the process);
Control (engineering or management controls to reduce the risk);
Can safe systems of work (SSW) make the task safer;
This process is known as the hierarchy of control and can easily be remembered by using the acronym ERIC:
- Eliminate
- Reduce
- Isolate
- Control
Posted by www.poolplantcourses.org. Posted In : Health & Safety
When Should I Backwash?
January 19, 2012Posted by www.poolplantcourses.org. Posted In : Frequently Asked Questions
Backwash
January 18, 2012It is a fairly simple and straight-forward process, and there should be a step-by-step guide contained within your normals operating procedures or systems of work etc. to guide you through it. For the pool plant operator, it is recommended that they get to know their pool plant system well enough that, eventually, they will not need to refer to a guidance document in order to properly perform a backwash. A very simplified, generic backwash description is given below:
1. Switch off circulation and chemical dosing.
2. Adjust valves so that the pool water goes into the filter at bottom and out at top, and from there to drains.
3. Switch circulation back on and leave running until pool water is visibly clear.
4. Switch circulation off.
5. Adjust valves so that the pool water goes in at the top and out at the bottom, but still runs to drains.
6. Switch circulation back on and leave running for a couple of minutes (this is called the 'rinse'.
7. Switch circulation off.
8. Adjust valves so that the pool water goes in at the top and out at the bottom, but this time returns to the pool.
9. Switch the circulation back on.
10. Open the air release valve to purge the system of trapped air.
As previously mentioned, this is a very watered-down (excuse the pun) description of the process. Other factors need to be considered such as air scouring, backwash velocity and filter pressure differential.
Posted by www.poolplantcourses.org.


