What is pH and Why is it so Important?
Posted by poolplantcourses.com on Monday, April 25, 2016 Under: Pool Water Chemistry

pH stands for the 'power of hydrogen' and is a critical factor in the treatment of pool water. The recommended range for the pH level to be maintained at is 7.2 - 7.4. The reason that the pH level needs to be kept between these values is that the disinfection efficiency of the chlorine falls off significantly at higher pH levels and the coagulant will also not be as effective. At lower pH values, the pool water will be too corrosive.
The effect of the pH level on the disinfection process is an area that many pool plant operators fail to fully understand. Therefore, they don't take the correct actions and end up with low quality swimming pool water and an excessive yearly spend on chlorine. Let's take a look at what's going on with pH and chlorine:
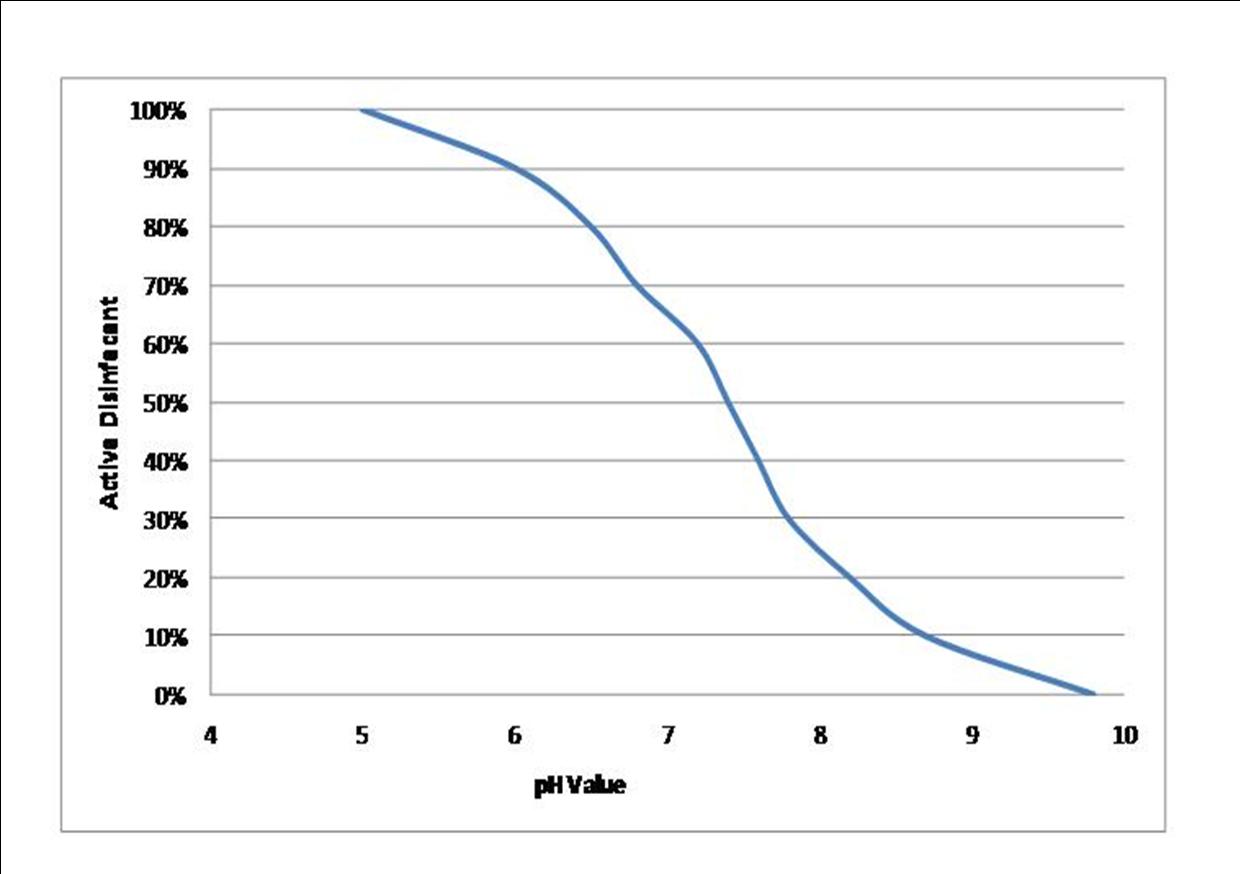
The blue line is the percentage of active disinfectant in the chlorine and as you can see, the percentage of active disinfectant is dependant on the pH level. When you add chlorine to the swimming pool water, chemical reactions start to occur. The chlorine reacts with the water and ends up producing hypochlorous acid and hypochlorite. The key disinfectant in chlorine is hypochlorous acid, which is about x100 stronger than the hypochlorite, so that's what we want more of. The higher the pH level, the higher the proportion of hypochlorite, the lower the pH level, the higher the proportion of hypochlorous acid. At a pH level of 7.4, you've got about 60% of the chlorine as hypochlorous acid, so if your free chlorine reading was 1.0 mg/l when tested, in real terms the amount of active disinfectant would only be around 0.6 mg/l. If the pH level was allowed to get to 7.8, then only 30% of the chlorine would be hypochlorous acid, so if the test reading came out at 1.0 mg/l again, the actual amount of active disinfectant would only be 0.3 mg/l, which would be too low for adequate disinfection.
You need to bear in mind that the free chlorine reading includes both the hypochlorous acid and the hypochlorite, but it does not tell you the proportion of each. This is why it's so important for pool plant operators to understand how and why the pH levels have such a dramatic effect on the disinfection process.
In : Pool Water Chemistry
